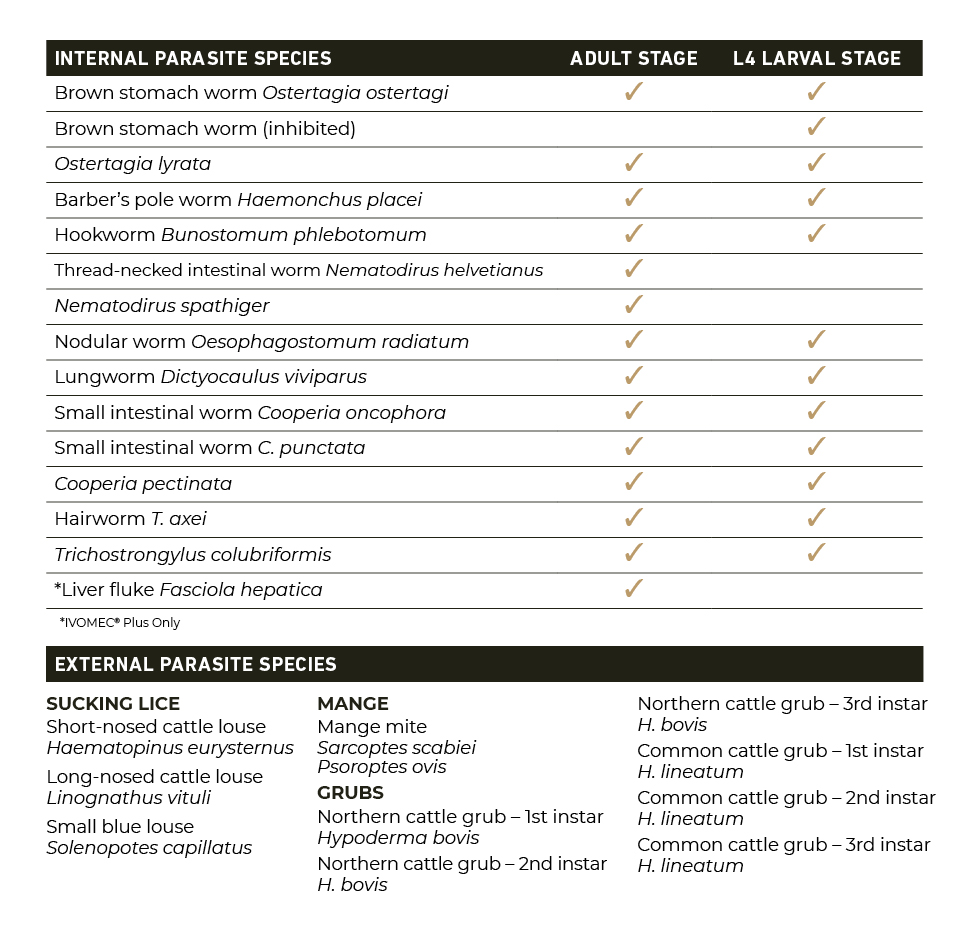
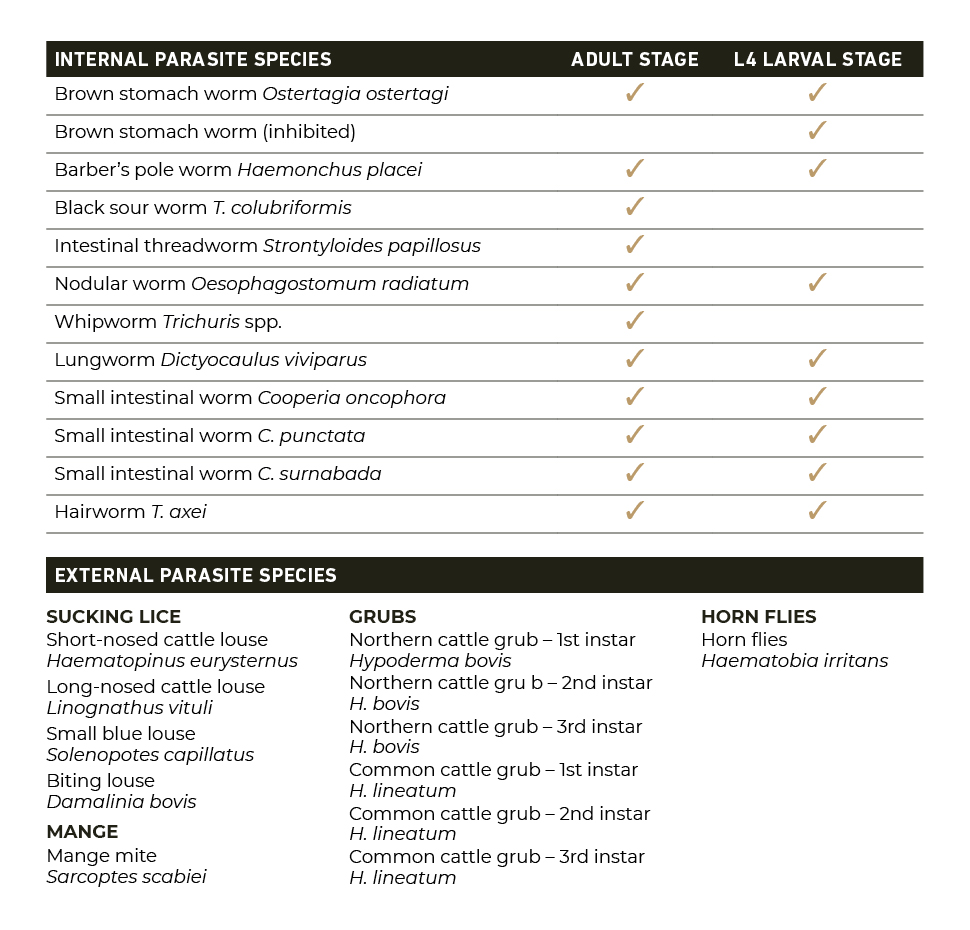
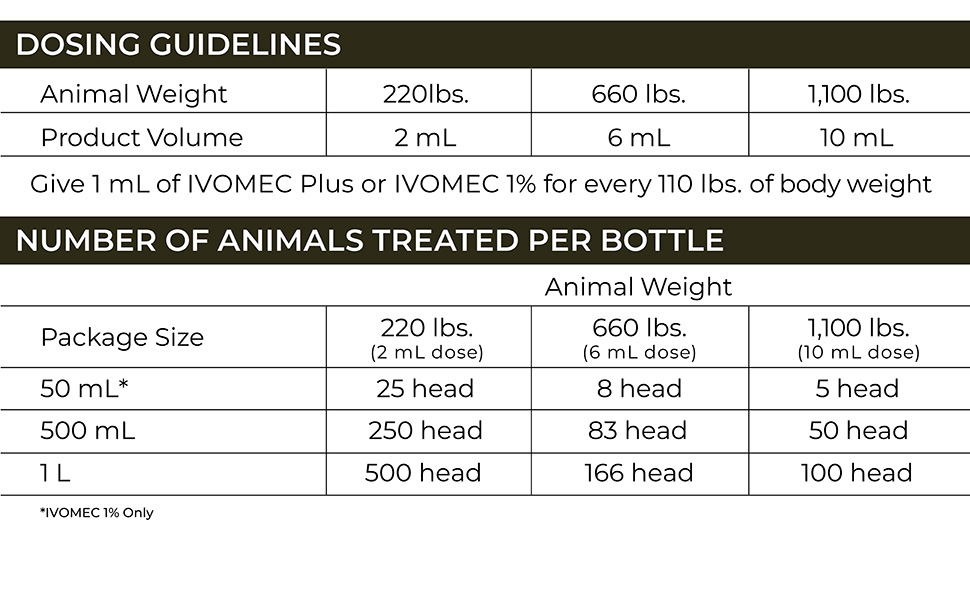
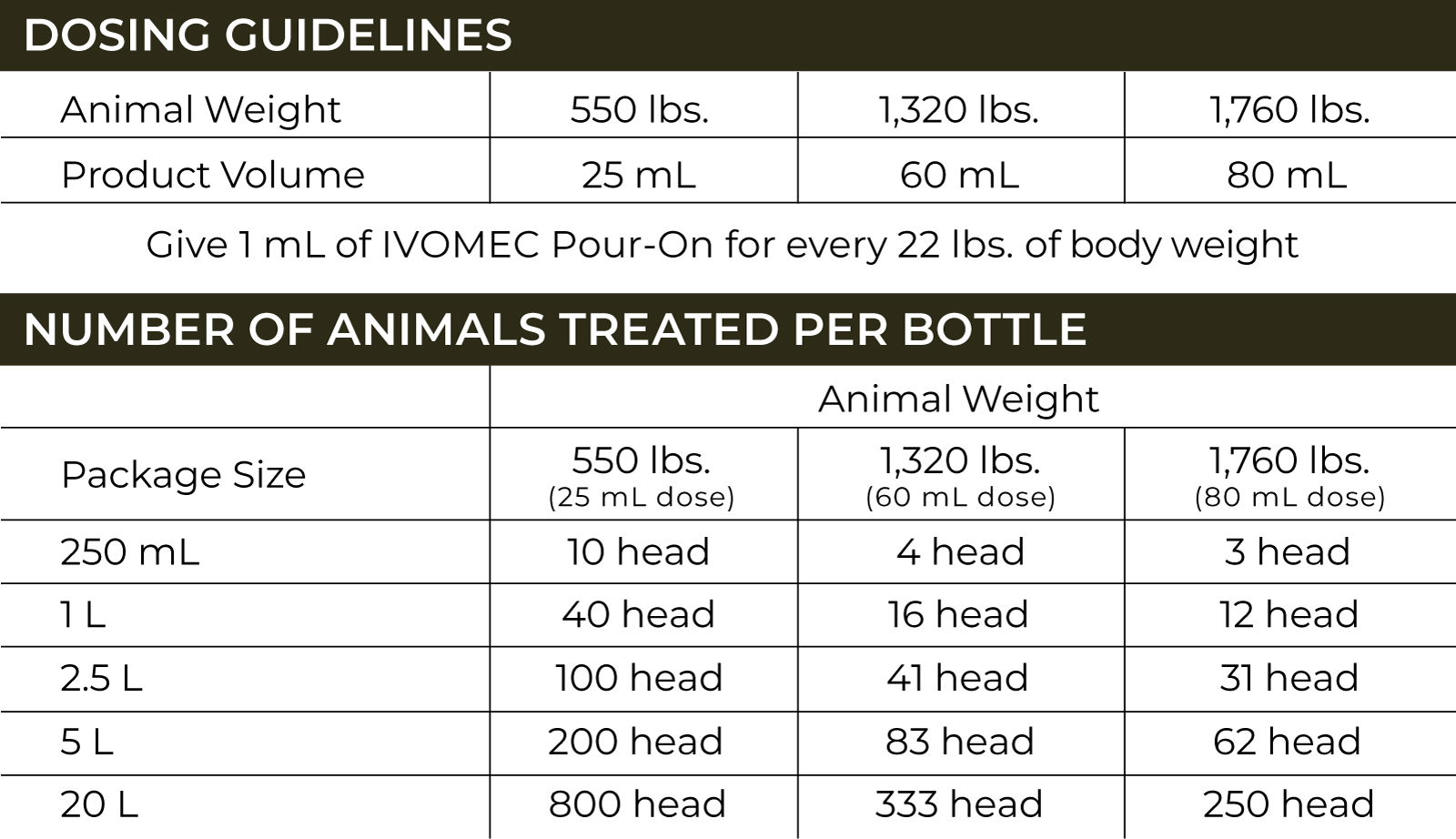
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the emergency use of the approved product IVOMEC® (ivermectin) 1% Injection for the prevention of infestations caused by New World screwworm (Cochliomyia hominivorax) larvae (myiasis) when administered within 24 hours of birth, or at the time of castration or the appearance of a wound in cattle, except for female dairy cattle producing milk for human consumption and calves that will be processed for veal.5 IVOMEC 1% Injection is not approved or conditionally approved for this use.
IVOMEC 1% Injection is approved for other uses. For additional information on the EUA, please refer to the IVOMEC 1% Injection NWS Fact Sheet and Prescribing Information.