EQUIOXX® (firocoxib)
The pioneer COXIB class NSAID with a decreased potential for risks commonly associated with nonselective NSAIDs.1

EQUIOXX for horses controls pain and inflammation associated with osteoarthritis for up to 24 hours with each dose.
A horse’s joints often need medical support in order to relieve the pain and inflammation associated with equine osteoarthritis. Equine osteoarthritis can happen in any age and any discipline due to everyday wear and tear. It is permanent and can lead to early retirement and quality of life issues in horses.
EQUIOXX is a COXIB class non-steroidal anti-inflammatory for horses that helps to manage the roller-coaster effect of pain and pain relief that can occur with other products that might require multiple daily dosing.
EQUIOXX tablets are small and palatable enough to be hand-fed with or without feed.
As with any prescription medication, prior to use, a veterinarian should perform a physical examination and review the horse’s medical history. A veterinarian should advise horse owners to observe for signs of potential drug toxicity. Use with other NSAIDs, corticosteroids or nephrotoxic medication should be avoided.
Features & Benefits
- Proven efficacy and a safety profile supported by science
- Each dose controls pain and inflammation associated with osteoarthritis in horses for up to 24 hrs
- EQUIOXX has been tested on more horses in safety studies than any other NSAID
- The first cyclooxygenase-inhibiting (COXIB) class nonsteroidal anti-inflammatory drug (NSAID) approved for horses
- Available as a chewable tablet
EQUIOXX can only be used by or on the order of a veterinarian. Once you have obtained a prescription, EQUIOXX is made for easy administration by an owner.
The recommended dosage of EQUIOXX Tablets is one 57 mg tablet administered orally to horses weighing 800-1300 lbs, (0.04-0.07mg/lb or 0.09-0.15 mg/kg), once daily for up to 14 days.
A veterinarian should advise horse owners to observe for signs of potential drug toxicity. As a class, nonsteroidal anti-inflammatory drugs may be associated with gastrointestinal, hepatic and renal toxicity. Use with other NSAIDs, corticosteroids or nephrotoxic medication should be avoided.
For more information please read EQUIOXX Tablets Product Insert.
As with any prescription medication, prior to use, a veterinarian should perform a physical examination and review the horse’s medical history. A veterinarian should advise horse owners to observe for signs of potential drug toxicity. As a class, nonsteroidal anti-inflammatory drugs for horses may be associated with gastrointestinal, hepatic and renal toxicity. Use with other NSAIDs, corticosteroids or nephrotoxic medication should be avoided. EQUIOXX has not been tested in horses less than 1 year of age or in breeding horses, or pregnant or lactating mares.


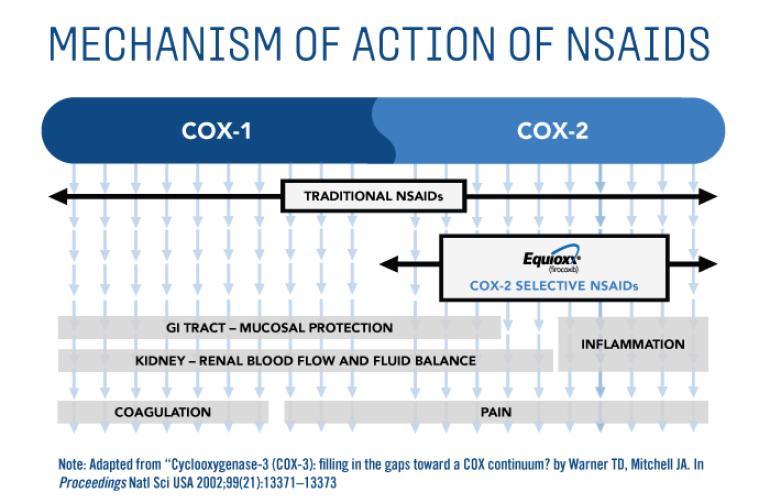
How EQUIOXX® (firocoxib) works
EQUIOXX selectively blocks the COX-2 isoenzyme which is the form that generates the prostaglandins mostly responsible for pain and inflammation. Yet it spares cyclooxygenase 1-isoenzyme (COX-1), which helps maintain normal body functions such as coagulation, gastric mucosal protection and blood flow to the kidneys.2,3,4
1 Kvaternick V, Pollmeier M, Fischer J, Hanson PD. Pharmacokinetics and metabolism of orally administered firocoxib, a novel second-generation coxib in horses. J Vet Pharmacol Ther 2007;30(3):208–217
2 Data on file at Boehringer Ingelheim. Clinical Experience Report PHN 471, PR&D 0030701.
3 Data on file at Boehringer Ingelheim, Clinical Experience Report PHN 471, Target Animal Safety Evaluation, Firocoxib (ML-1, 785, 713) Oral Paste for Horses
4 Warner TD, Mitchell JA. Cyclooxygenase-3 (COX-3): Filling in the gaps toward a COX continuum? in Proceedings Natl Acad Sci USA 2002;99(21):13371–13373.
EQUIOXX® is a registered trademark of Boehringer Ingelheim Animal Health USA Inc. ©2025 Boehringer Ingelheim Animal Health USA Inc., Duluth, GA. All rights reserved.
US-EQU-0116-2021-V3