LEGEND® (hyaluronate sodium)
First hyaluronic acid joint therapy for intravenous and intra-articular administration approved by the FDA
LEGEND Injectable Solution is indicated in the treatment of joint dysfunction of the carpus or fetlock in horses due to non-infectious synovitis associated with equine osteoarthritis. Equine osteoarthritis can happen in any age and any discipline due to everyday wear and tear.
The active ingredient in LEGEND, hyaluronic acid (HA), decreases the presence of inflammatory mediators in the joint, improves synovial fluid quality, and reduces the degree of lameness.2,3,4 These improvements are seen whether LEGEND is administered by intravenous (I.V.) or intra-articular (I.A.) injection. LEGEND hyaluronate sodium injectable is sterile, pyrogen-free and approved by the FDA.
Not for use in humans. Keep this and all other drugs out of reach of children. The safety of LEGEND has not been evaluated in breeding stallions or in breeding, pregnant or lactating mares. LEGEND 4 mL and LEGEND Multi-Dose Injectable Solution are administered by intravenous injection only.
Features
- Joint therapy approved by the FDA for both intravenous and intra-articular use, allowing treatment tailored to the veterinarian's preference and the horse's individual needs
- Millions of doses sold5
- More than 25 years of treatment success
- Safe and effective, proven by multiple studies
- Reduces production of inflammatory mediators, joint inflammation and the resulting lameness for at least 45 days after being given once a week for three weeks4
Benefits
- Safe and effective
- 90% of cases treated intravenously judged to have excellent to good overall clinical improvement1
- 96% of horses treated intra-articularly were judged to have excellent to good overall clinical improvement1
- Improvement can be seen after one dose
- Breaks the cycle of inflammation and lameness associated with synovitis
- Stimulates endogenous hyaluronic acid production2
- Decreases the presence of inflammatory mediators4
- Easy to use I.V. administration for when intra-articular administration is not practical or desired
LEGEND can only be used by or on the order of a veterinarian.
LEGEND Multi Dose (20 mL) and LEGEND 4 mL (40 mg) can be injected intravenously only at a dosage of 4 mL. Intravenous treatment may be repeated at weekly intervals for a total of 3 treatments. LEGEND 2 mL (20 mg) can be injected intravenously (at a dosage of 4 mL) or intra-articularly in the carpus or fetlock.
Not for use in humans. Keep this and all other drugs out of reach of children.
For more information please read Legend 2 mL Product Information, Legend 4 mL Product Information, and Legend Multi-dose 20 mL Product Information.
Federal law restricts this drug to use by or on the order of a licensed veterinarian. For use only in horses. Do not use in horses intended for human consumption.
LEGEND 4 mL and LEGEND Multi-dose 20 mL are approved only for I.V. use. LEGEND 2 mL is approved for I.V. and I.A. use. The intra-articular safety of LEGEND Multi Dose 20 mL with benzyl alcohol has not been evaluated.
The safety of LEGEND has not been evaluated in breeding stallions or in breeding, pregnant or lactating mares.
The following adverse reactions have been reported following use of LEGEND Injectable Solution: Following intravenous use: occasional depression, lethargy, and fever. Following intra-articular (LEGEND Injectable Solution — 2 mL only) use: lameness, joint effusion, joint or injection site swelling and joint pain.
Not for use in humans. Keep this and all other drugs out of reach of children. Strict aseptic techniques should be observed when administering by intra-articular injection.
Store at or below 77° F (25° C). Brief excursions to 104° F (40° C) are permitted.
Flexible Treatment.
Equally Effective.
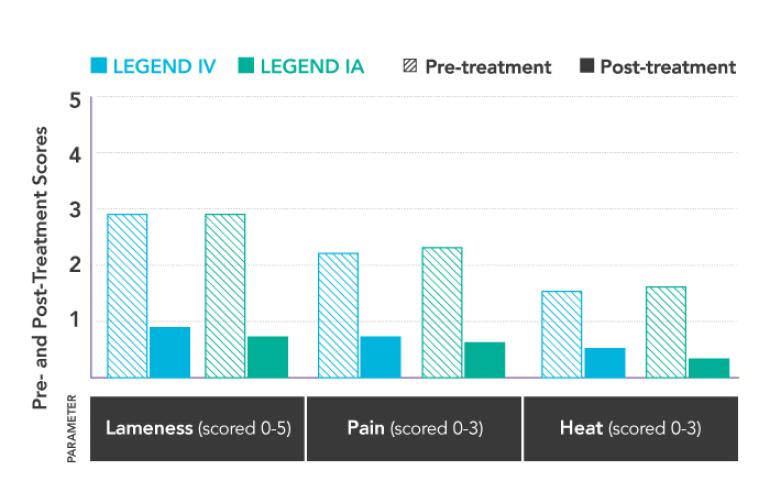
Equally Effective.
A blinded clinical study compared the treatment effectiveness of I.V. or I.A. administration of LEGEND in 46 horses. Both groups showed significant improvement. LEGEND-administered I.V. had no statistical difference in any of the measured parameters from LEGEND-administered I.A.1
1 LEGEND Product Label
2 Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int 1987;7(3):113–222.
3 McIlwraith CW. Traumatic joint disease. Orthopaedic Research Center, Colorado State University website http://csu-cvmbs.colostate.edu/academics/clinsci/equine-orthopaedic-research-center/orthopaedic-topics/Pages/traumatic-joint-disease.aspx#legend. Accessed July 31, 2015.
4 Kawcak CE, Frisbie DD, Trotter GW, et al. Effects of intravenous administration of sodium hyaluronate on carpal joints in exercising horses after arthroscopic surgery and osteochondral fragmentation. Am J Vet Res 1997;58(10):1132–1140.
5 Data on file at Boehringer Ingelheim
LEGEND® and the Horse logo® are registered trademarks of Boehringer Ingelheim Animal Health USA Inc. ©2025 Boehringer Ingelheim Animal Health USA Inc., Duluth, GA. All rights reserved.
US-EQU-0115-2021-V3