by Boehringer Ingelheim/March 31, 2022

Porcine circovirus type 2 (PCV2) is one of the most economically significant viruses in swine production. While several genotypes have been identified, the industry has observed a clear shift over the past decade, with PCV2d overtaking PCV2a to become the predominant genotype in the United States and globally.1-3 Since the emergence of PCV2d, the U.S. swine industry has relied primarily on PCV2a based vaccines to provide cross-protection against PCV2d. While these vaccines have demonstrated efficacy in preventing clinical porcine circovirus associated disease (PCVAD), they do not completely prevent viral replication. Breakthrough infections continue to occur and coinfections, particularly with porcine reproductive and respiratory syndrome virus (PRRSV), may enhance viral replication and immune suppression, thus worsening PCVAD severity.
Study Key Findings
- Severe co-infection of porcine circovirus type 2 (PCV2d) and porcine reproductive and respiratory syndrome virus (PRRSV) resulted in mortality of more than 60% of non-vaccinates, compared to only 8.2% of PCV2d vaccinates.4
- PCV2d vaccinates demonstrated a significant reduction in lymphoid lesion severity and viral replication under severe PCVAD field conditions compared to non-vaccinates and PCV2a vaccinates.4
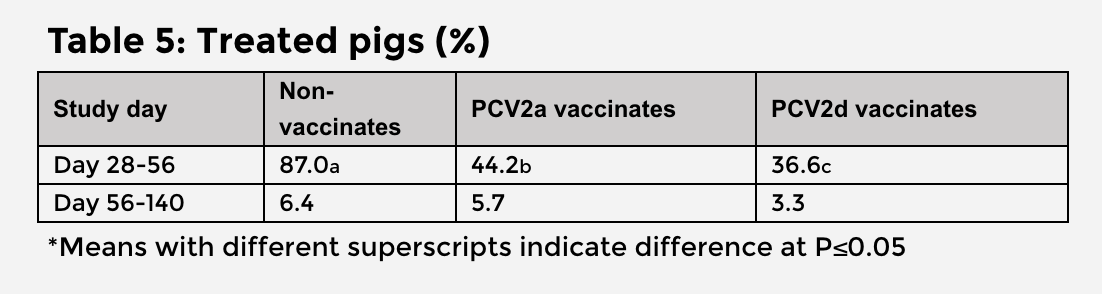
- The number of pigs requiring treatment in the acute phase of PCVAD was significantly fewer among PCV2d vaccinates compared to non-vaccinates and PCV2a vaccinates.4
Study Design
Overview
This randomized, blinded challenge study measured whether a PCV2d-based vaccine would provide more robust and targeted protection against PCVAD in cases of severe PCV2d co-infections (PRRS, influenza A virus in swine [IAV-S] and endemic bacteria) compared to cross-protection levels of a PCV2a-based vaccine.
Study Design
The study consisted of 1,410 conventional production pigs, randomized and blocked based upon weight and gender, into three groups on day 0: 470 non-vaccinated controls, 470 PCV2a vaccinates and 470 PCV2d vaccinates. On day 28, all groups were inoculated with 1 mL IM and 1 mL IN of PCV2d, as well as 2 mL IM of PRRSV 1-7-4. Mortality, percentage of treated pigs and body weights were recorded on days 0, 28, 56 and 140. On day 56, 20 pigs from each group were euthanized to evaluate gross lesions, lymphoid changes, amount of virus in tissue and viremia levels.
Study Results
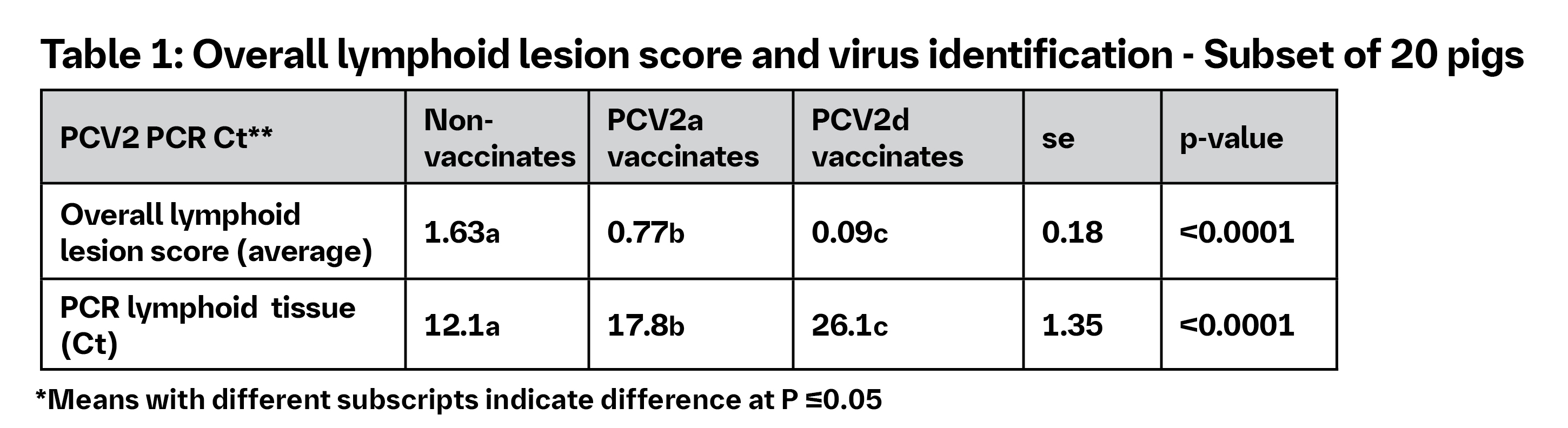
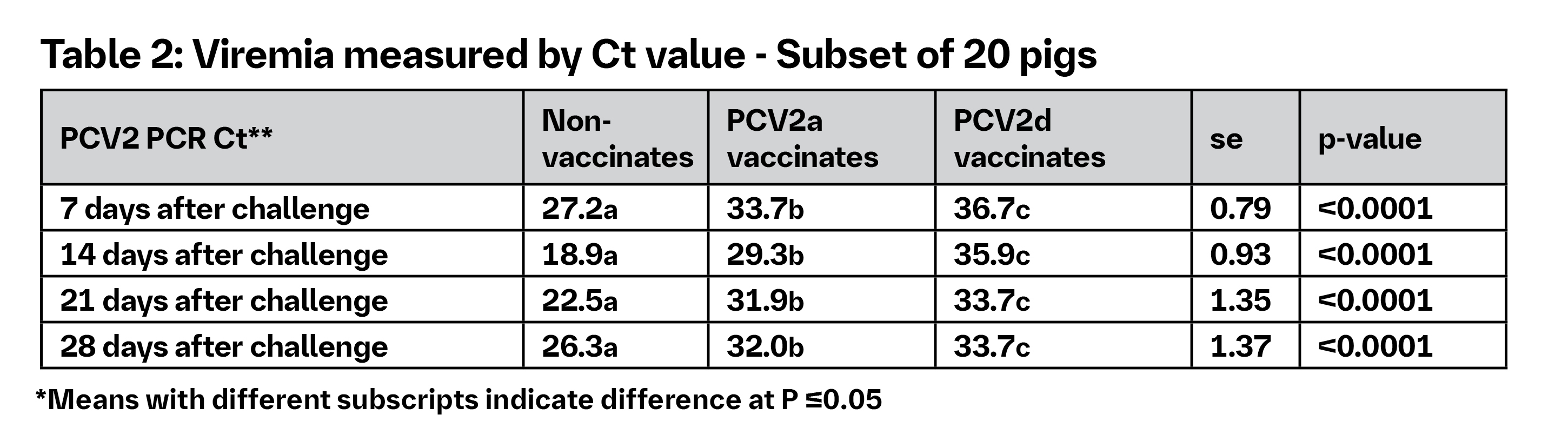
Experimental co-infection resulted in high mortality and severe PCVAD among non-vaccinates. Table 1 shows the effect of overall lymphoid lesion score and the detection of the virus. Viremia results are highlighted in Table 2.4
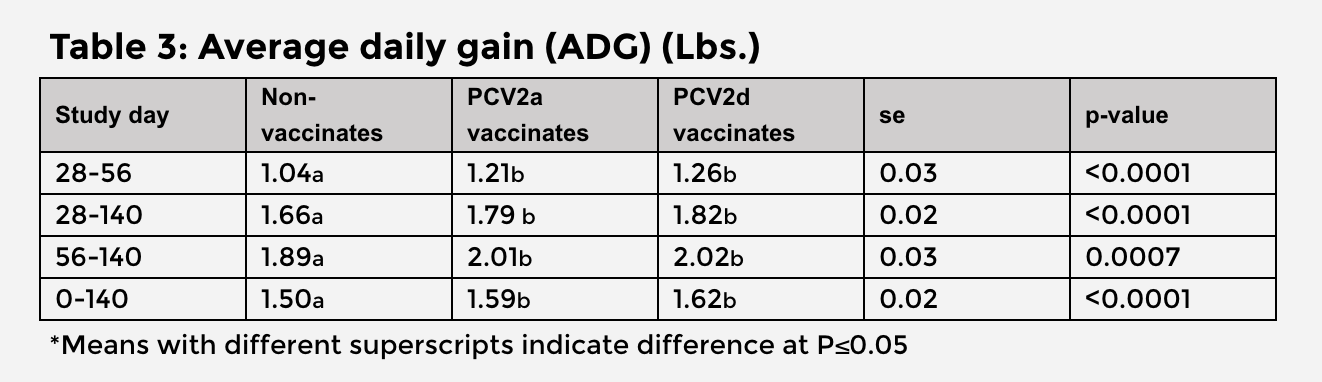
Average daily gain (ADG) was significantly higher among both vaccinated groups compared to non-vaccinates (Table 3). ADG was 0.03 lb./day higher among PCV2d vaccinates than PCV2a vaccinates.4
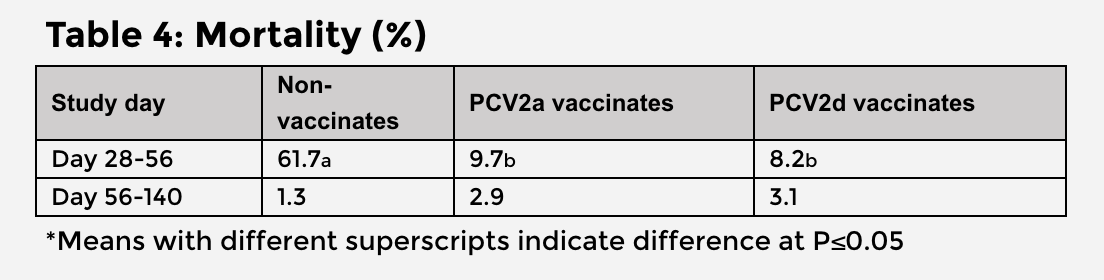
There were no significant differences in mortality between the vaccinated groups (Table 4). The PCV2d vaccinates had 1.5% lower mortality and 7.6% fewer treated pigs than PCV2a vaccinates.
Conclusion
Under severe PCVAD conditions, PCV2d vaccinates demonstrated a reduction in lymphoid lesion severity and viral replication (serum and tissue) compared to PCV2a vaccinates. Both vaccinated groups had significantly higher ADG and significantly lower mortality rate and number of treatments than non-vaccinates.
These findings underscore the need for optimized vaccination strategies, especially in the face of emerging PCV2 genotypes. For swine herds experiencing PCVAD associated with PCV2d, a vaccine containing a homologous PCV2d component presents the best option for optimal protection and herd health management.
References
1 Franzo G, Tucciarone CM, Legnardi M, Drigo M, Segalés J. An updated phylogeography and population dynamics of porcine circovirus 2 genotypes: are they reaching an equilibrium? Front Microbiol 2024;15:1500498.
2 Kroeger M, Fano E, Sponheim A, Schwartz K, Leite F, Gomez-Duran O, Lecznieski L, Piñeyro P. Assessment of homologous and heterologous PCV2 vaccine efficacy in a PCV2d/PRRSV co-challenge model. Vaccine 2025;60:127303.
3Kroeger M, Fano E, Sponheim A, Schwartz K, Leite F, Gomez-Duran O, Lecznieski L, Piñeyro P. Enhancing PCV2 control: Homologous vs heterologous vaccination in coinfection scenarios. In Proceedings. AASV 2025.
4Fano E, et al. Exploring the efficacy of a PCV2d-based vaccine under current severe PCVAD conditions, in Proceedings. AASV 2022.
©2025 Boehringer Ingelheim Animal Health USA Inc., Duluth, GA. All rights reserved. US-SWN-0018-2025