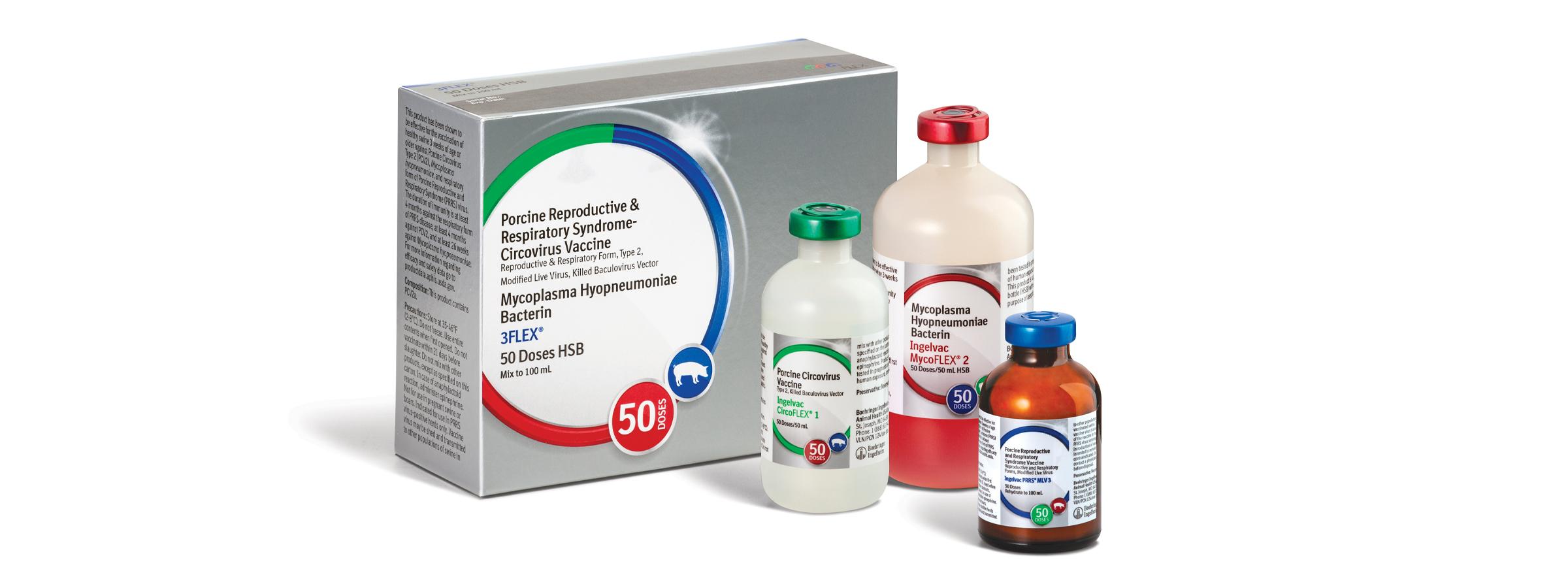
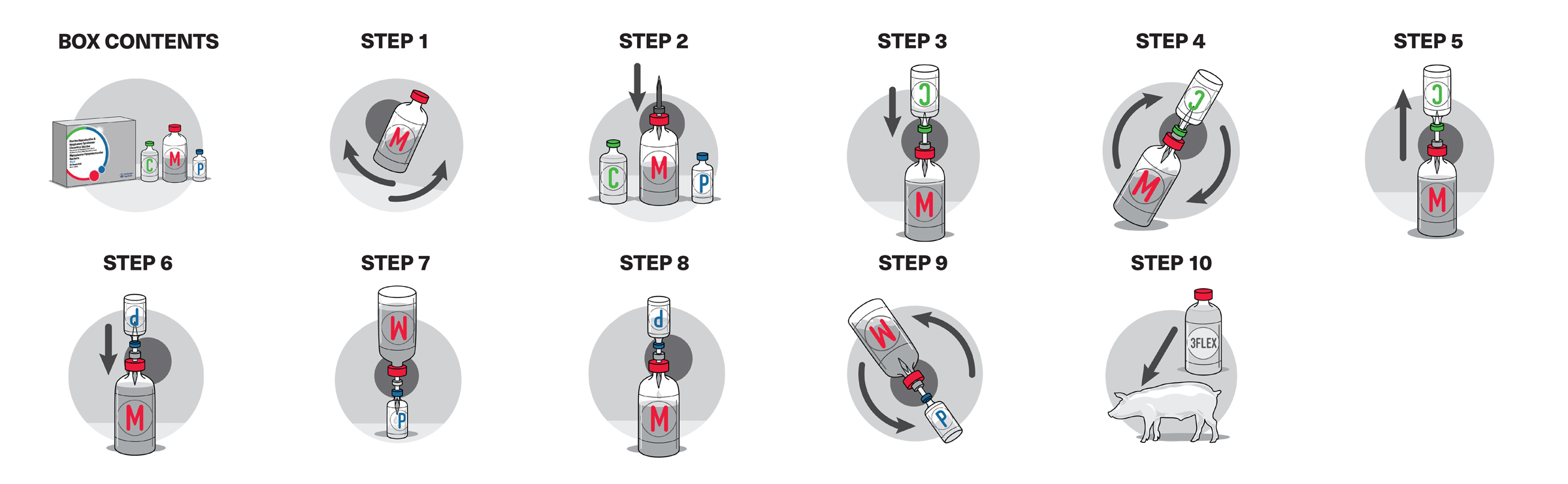
IMPRANFLEX is a proprietary water-based, sponge-like matrix adjuvant found in all FLEX Family vaccines that offers the following advantages:
- Does not contain and is not derived from any material of plant or animal origin
- 100 percent oil free
- Low viscosity and good syringeability
- Very safe for farm staff and animals
- Less stressful for the animals
- Non-virucidal to PRRSV