INGELVAC PRRS® MLV
Porcine Reproductive and Respiratory Syndrome (PRRS) Vaccine
FLEXIBLE: The only PRRS vaccine licensed by the USDA for use in the widest range of combinations (for example: 3FLEX®, FLEX CIRCOPRRS® and FLEX MYCOPRRS®) to best meet the needs of your protocol and operation.
RELIABLE: Aids in the prevention of respiratory disease, and aids in the reduction of reproductive disease caused by PRRS virus. Proven protection against current and relevant strains, like PRRS 1-4-4 L1C.1
SAFE: Reduced magnitude and duration of viremia, as well as reduced shedding of wild-type PRRSV within and from vaccinated populations.
Consistent high immunogenicity and proven cross-protection against heterologous challenge (including Lineage 1 viruses).
Safe in pigs, gilts and sows at any stage of production.
Easy to use, cost effective and field-proven against respiratory and reproductive forms of PRRS virus.
Whole-herd or population-based PRRS control program advantages.
Gilt stabilization/acclimatization program advantages.
Customized control allows flexible vaccination scheduling (multiple combinations).
Significantly reduces reproductive failure due to PRRS virus.
Mitigates consequences of infection and improves health and performance.
Reduces levels of wild-type PRRSV within and from vaccinated populations over time, decreasing the risk of virus exposure.
- Enables vaccinated pigs to perform similarly to unchallenged pigs at lower levels of challenge.
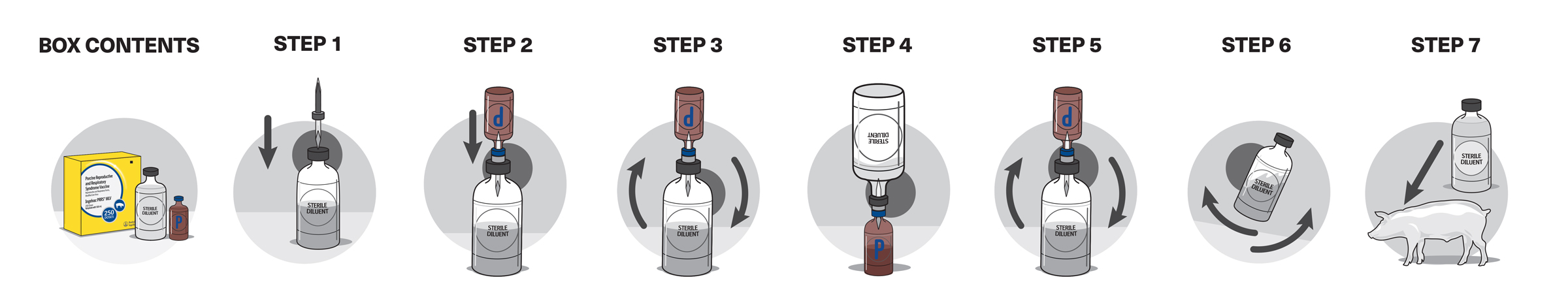
Mix the package contents according to label instructions.
Using aseptic technique, inject a single 2-mL dose of vaccine intramuscularly (same dose size (2 mL) for pigs, adult breeding-age or pregnant females).
Does not need to be tempered.
Revaccination of the whole herd can occur every three to four months or as directed by your veterinarian.
INGELVAC PRRS MLV Mixing Video
Product Inserts & Labels
INGELVAC PRRS MLV Safety Data Sheet (SDS)
Store in dark at 35-46ºF (2-8ºC).
Do not freeze.
Use entire contents when first opened.
Do not vaccinate within 21 days before slaughter.
In case of anaphylactic reaction, epinephrine is symptomatic treatment.
Do not vaccinate boars.
Indicated for use in PRRS virus–positive herds according to label direction.
Not recommended for use in PRRS negative (naïve) herds.
- For veterinary use only; for use only in swine as directed.
Custom Transfer Spikes
For proper mixing of the FLEX Family of products, we recommend the use of a vented transfer spike. (A traditional transfer needle cannot be used.)
The PrimaTech® - Neogen® transfer needle is provided for INGELVAC PRRS vaccines. To request transfer needles or for more information about which needle to use, please contact your Boehringer Ingelheim key account manager.

1 Philips R, Fiechtner J, Haiwick G. Efficacy evaluation of INGELVAC PRRS® MLV against a PRRSV 1-4-4 L1C.5 variant challenge. In Proceedings. Amer Assoc Swine Vet 2025.
INGELVAC PRRS®, 3FLEX®, FLEX CIRCOPRRS®, FLEX MYCOPRRS® are registered trademarks of Boehringer Ingelheim Vetmedica GmbH, used under license. All other trademarks are the property of their respective owner. ©2023 Boehringer Ingelheim Animal Health USA Inc., Duluth, GA. All rights reserved.
US-SWN-0045-2025